Quantitative Imaging in Oncology
Quantitative imaging in oncology combines advanced microscopy, image analysis and machine learning to study cancer in unprecedented detail. By extracting rich spatial and molecular information from tissues, we aim to better understand how tumors grow, respond to treatments and interact with their surroundings. In our lab, we have developed colocatome frameworks to map and quantify in situ cellular organization, revealing how microenvironments regulate cell behavior. In parallel, we extract reproducible radiomic features such as texture, intensity and shape, together with quantitative MRI metrics like relaxation times, and apply interpretable machine-learning models to link these imaging biomarkers to clinical outcomes. This enables precise tumor localization and non-invasive monitoring of disease progression.
Quantitative Imaging and Spatial Analysis
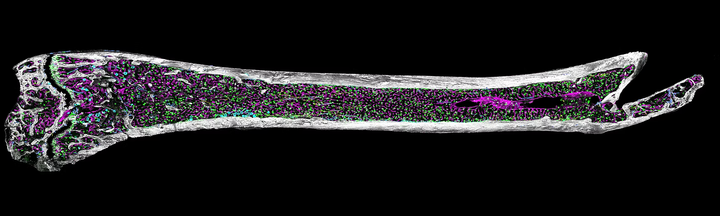
Our work focuses on developing computational frameworks to analyze high-resolution multiplex microscopy images of tissues such as bone marrow, generated by Quantitative Spatial Cancer Biology - Kokkaliaris lab. We specialize in extracting spatial and morphological features from complex, multi-modal image data.
We have developed a framework that enables the integration and analysis of multiple biological replicates with complementary information in a shared spatial reference space. Building on this, we established a pipeline to extract and quantify spatial remodeling of the cellular neighborhood during the aging process. These tools allow us to investigate how hematopoietic stem cells, blood vessels, megakaryocytes, adipocytes, and stromal components are spatially organized and how these patterns evolve with age or in response to treatment.

Radiomics and Quantitative MRI
Our imaging research also includes radiological data analysis, with a focus on transitioning from qualitative interpretation to quantitative, reproducible metrics. In radiomics, we extract features such as texture, intensity, and shape from defined regions of interest within MRI scans, and use machine learning models to link these features to clinical outcomes. We emphasize interpretability and robustness, testing models on controlled environments to avoid confounding due to real-patient variability and ethical exposure limitations.
In parallel, we employ quantitative MRI (qMRI) techniques to move beyond traditional contrast-based imaging. By measuring intrinsic physical properties of tissues, e.g. relaxation times, we obtain microstructural insights into tissue composition, particularly in brain imaging. Combining qMRI with machine learning enables precise localization of tumors and assessment of disease progression, further enhancing the clinical utility of radiological data.